A new style of commits?
I thought since I hadn’t pushed an actual page in quite literally ages I might just make a few notes on some things I’m thinking about. It would’ve usually been Literature (it is literally everything that cooks in my head, everything else simmers), but hopefully this new styles of a small commit every week will work given the crazy stuff looking ahead (and also to calm the voices).
A little disclaimer on this though, some of the topics don’t at all pertain to what we’re doing, but just kinda funny so I think about them… hence I write about them here.
For today’s little commit, it’s… Chemistry and Physics! (well I think I just will be talking about them for every one of these save for a few Lit or funny Spec commits here and there). Specifically the things swirling round right now is in reference to titrations, resonance structures and bosons (bozons??). I promise they will make a lot more sense when I talk about them, and if not… I just need this for my commits.
Anyhoo
Rinsing materials used in Titrations
Titrations is chock-full of SIS (science inquiry skills). Even one slight error throws the whole process, which usually would be volumetric analysis, into disarray. Of course, the reason being that chemists or lab technicians or anyone else in a similar field would like to know the exact concentration of some solution.
Even washing and rinsing the materials beforehand is meticulously done so that one bit of error can affect the process, and this is where my thoughts begin on this. A certain… blue gradient guy… got a bit confused about this so I’ll spell it out a bit to make sure you guys don’t 👍
Though the acid or base in the titration can be interchangeable, in the context of our course, and traditionally, the acid, in the burette, is usually added to the base, which is in the conical flask with indicator added.
Also I’ll be assuming you know the rest other than what I say here (because it’s rather abridged and only focuses on the rinsing solutions and why they work).
There are 4 materials in question that have to washed with specific liquids or solutions, being:
- Volumetric flask (what you put the solution whose concentration you don’t know, named the analyte, in, then make a specific volume of, that is usually 250 mL in our case)
- This must be washed with distilled (or deionised) water. The reason is that this will remove any impurities or other compounds in the flask, that may be present due to the flask being used for a prior titration or the water being used to wash it prior having impurities. It will not affect its usage, as this is the same water you will be adding when diluting the analyte to a volume of 250 mL.
- Pipette (what you take specific samples of volume of the analyte from the volumetric flask, known as aliquots)
- This must be washed with initially distilled water the same solution as the analyte that you are taking aliquots of.
- Burette (a tube that can pour in the titrant slowly so that measured volumes can be delivered to the conical flask where the other solution is)
- This also must be washed with distilled water first, then the same solution as the titrant that you will be adding to the conical flask.
- Conical flask (where the neutralisation reaction of the titration occurs)
- Now this…must be washed with distilled water and distilled water only. The reason being, you also want to remove any impurities that may be in the flask, similar to the volumetric flask. As to why it does not affect the process, what we are looking to reach in our conical flask is stoichiometrically equal moles of the titrant and analyte (or in our context, the acid and the base). It doesn’t matter if the concentration of titrant or the analyte is changed by having a bit of extra distilled water remaining after you rinse the conical flask because the moles of the titrant and analyte stay the same. A reaction (for this context) is about moles, not concentration.
In any case, if you want to learn more about titrations in a rather informal matter, just look to the crazy guy who commented on some Minecraft Youtuber’s video detailing how titrations work that Ludwig went through in his video (I’m talking to you ninepointcookie).
Resonance structures
So you know how the Lewis diagrams for carbonate ions and nitrate ions were slightly hard to draw sometimes? Well don’t worry, they’ll get even more confusing!
How we know it is that Lewis diagrams can be used to detail how separate atoms in certain molecules, ions or compounds are bonded together. This, of course, is primarily the method to find the types of covalent bonds for certain molecules, which can be done by knowing their formal charges (or at least I do it by using formal charges), using an atom’s already existing electron configuration to intuitively determine how it will bond with another atom by tending towards a full valence electron shell and sharing the same number of electrons. For example, carbon has 4 valence electrons, and requires 4 more valence electrons to have a full valence shell, thereby requiring 4 bonds to reach a full valence shell (because the 4 more electrons it can get can only be through covalently bonding with another atom and it can’t have a lone pair because it needs to share the same number of electrons as another atom shares with it, yada yada yada).
Well, you might remember that there was at least one double bond and two single bonds in the carbonate or nitrate ions. This little guy (haha get it it’s smaller than a single bond… I am losing my mind) can between any oxygen atom and the central atom (either carbon or nitrogen). You might’ve assumed that was because the ion could just rotate around the central atom and you’d get the same thing… but that’s not the case. If you were to try and experimentally determine the bond lengths of a carbonate ion and thus, prove that there is only one double bond and two single bonds… you would actually find out that all three are the same length! This must mean they have the same bonding type, but something else is a bit wrong as well. Single bonds have a length of 148 picometres (pm) while double bonds have a length of 120.7 pm, but the actual bond length is 129 pm. So what bonds are they? They aren’t exactly double bonds, but they aren’t exactly single bonds.
Remember how I mentioned that the double bond can exist between any oxygen atom and the central carbon atom? Considering this, we have three possible Lewis diagrams that just look like they’ve been rotated. But, instead of the carbonate ion being one of these structures the carbonate ion can exist in all three structures at the same time! These are known as resonance structures, because the carbonate ion resonates between all three structures. This creates a resonance hybrid which is close to how the actual structure of the ion, instead of being one of the Lewis diagram. This is due to the nature of the delocalised ions in the covalent bonds between the carbon atom and oxygen atoms, instead creating a structure that utilises all three Lewis diagrams as contributing structures, shown below.
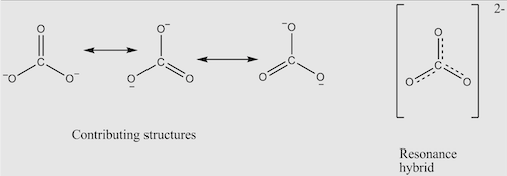
Do they oscillate between these structures? That’d be so cool. But I, uh, simply don’t know. I just learned about this quite recently and it popped up in my head and remembered how funny it was.
Love how my high school Chemistry teacher alluded to this when talking about benzene in organic chemistry (yes, resonance structures apply to the strange bonds in benzene too!). Also, another word for resonance structures is canonical forms… so… (if you know, you know)
The funny natural forces (and why almost every force is electrostatics)… but only gluons and photons
Ahh, nothing like a Physics test full of imaginary forces that we thought were actually forces of their own hundreds of years ago only to realise that they eventually derive into just electrostatics (Yes. Yes it does.). My standard model page isn’t even finished, so this little update can hopefully cover some stuff… since people actually wanted to learn about it.
So Physics really loves forces. A lot. So much so that when light was proved to both act like a wave and a particle, eventually, they decided that forces themselves could also be represented through particles. Not your reaction forces, friction forces, centripedal forces and other “fake” forces (I love saying that). The true, pure grade, four fundamental natural forces, being strong nuclear force, electromagnetic force, weak nuclear force and gravitational force (you’ll realise why I said them in that order soon). So, each of these forces were given their own particles, named bosons. Fermions, particles that govern matter (i.e. quarks, leptons (electrons and such), protons, neutrons etc.) interact with each other via the exchange of gauge bosons, fundamental (not being made of any smaller particles) particles that govern force. However, gauge bosons only represent three of these forces, strong and weak nuclear force and electromagnetism, due to them being based off of gauge principles that detail interactions between all these forces only. Gravity seemingly does not fit in because it is negligible at a sub-atomic level, while these forces are not. Yes, a graviton has been theorised, but hasn’t actually formed experimentally, and general relativity (i.e. how gravity works) not being applicable to quantum mechanics (how sub-atomic particles work) suggests something more is at play with gravity than just a particle.
For now, I’ll just detail gluons and photons because everything else is going to destroy the 90 line limit I’m putting for this commit. Also, it’s more on topic as to the what I meant by “fake forces”.
The boson for strong nuclear force is known as the gluon (Glue. Yes. Glue.), which as you may remember, is used to hold a nucleus together, overcoming the repulsion between the protons in the nucleus. You also might remember that part of the mass of the nucleons of the certain nucleus of an atom decays into energy, which is equal to the binding energy of the nucleus. Welllll… strong nuclear force originally acts in between quarks, sub-sub-atomic small particles that make up baryons such as protons and neutrons, sticking them together and acting like “glue” to form the nucleons in the first place. However, it doesn’t just stick quarks together, it sticks baryonic matter (i.e. protons and neutrons) together as well, further assisting in the creation of nuclei and overcoming the electrostatic repsulsion force between protons. Gluons and quarks cannot be free particles by themselves, and cannot exist without each other, so each quark comes along with its own glue(on).
The boson for electromagnetic force, or electrostatic force, (it’s quite complicated, but for now, know they are two sides of the same coin), is known as the photon. Photons? Light? Yes. Photons mediate the interactions between charged particles, acting as the force for electromagnetism. These in fact are exchanged between charged particles that get near each other; in nuclei, wires and magnets, anything that involves charges at all. So, when you push against a wall, a reaction force is felt by you, equal to the force that you are applying to the wall. This is because the delocalised electrons gathered at your hand and even the electrons in the atoms in the molecules in the compounds upon compounds in the cells that make up your skin, and the electrons in the atoms inside of the wall are repelling each other. Yes, the valence shell electrons between the very atoms that make up an object interact with the valence shell electrons of atoms in another object, causing a repulsion through electrostatic force. This is why you can’t simply phase through matter (yes, if electromagnetism didn’t exist, you can phase through anything; or if you or the object you’re trying to go through doesn’t have any particles that repel particles that you’re made up of or have on you, then you can also phase through that object). At a sub-atomic level, electrons are shooting photons at each other out of sync, pushing them farther and farther away from each other. On the other hand, inside of the nucleus, exchanges of photons between protons and electrons do occur, but occurs such that instead of the photons hitting the protons and electrons apart and causing them to have a repulsive electrostatic force, they each exchange a photon (as though timed) such that they both exchange and receive photons at the same time, attracting them. This is explained very well in ScienceClic’s video on electromagnetic fields and electric/magnetic forces, which can be found here.
In any case, that’s all from me today.
hee hee hee haw
If you’ve made it here, my blazing friend, then this is for you:
God bless true! True will never die ! Liers will be kicked off…